| Availability: | |
|---|---|
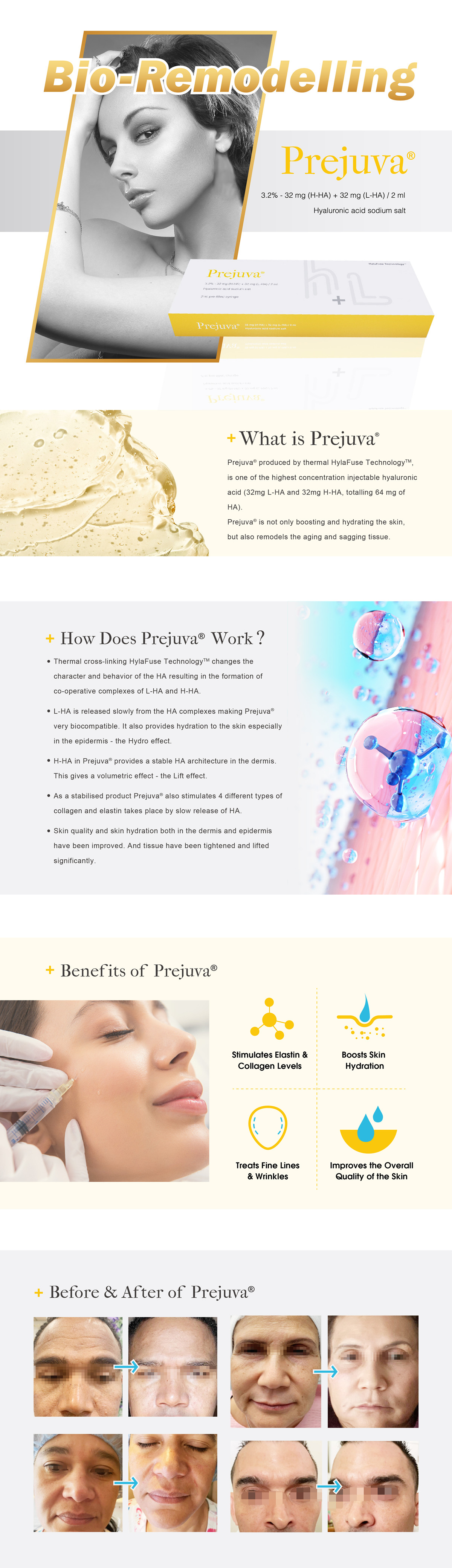
PREJUVA®

PREJUVA® constitutes a buffered physiological solution of high molecular weight (H-HA) and low molecular weight (L-HA) HA. The HA, at both high and low molecular weights used in the device, is obtained through a biofermentation process without chemical modification and therefore, resulting in excellent tolerability.
Furthermore, due to a specific and patented treatment of the solution (HYLAFUSE Technology TM), the H-HA and L-HA chains, contained in PREJUVA®, interact with each other providing unique rheological characteristics and thus allowing the administration of higher concentrations of HA without increasing the viscosity.
PREJUVA® acting through a corrective/filling action of natural and induced cutaneous depressions, intervenes:
• in the physiological process of skin aging, the effects of which include reduced skin hydration, the alteration of elastic fibers and collagen of the dermis, with loss of turgor and skin tone;
• in the dermal tissue repair process, in cases of scars resulting from superficial Cutaneous trauma (e.g. acne and chicken pox scars).

Dermax Co., Ltd. is a biotechnology enterprise specialized in the research, production and sale of aesthetic products. The establishment of professional technical team, GMP production plant, complete and strict testing system has guaranteed stable and advanced manufacturing technique. The high quality, integrity and global marketing network enable our company to win high praise home and abroad including in Europe, America, Southeast Asia etc.


PREJUVA® constitutes a buffered physiological solution of high molecular weight (H-HA) and low molecular weight (L-HA) HA. The HA, at both high and low molecular weights used in the device, is obtained through a biofermentation process without chemical modification and therefore, resulting in excellent tolerability.
Furthermore, due to a specific and patented treatment of the solution (HYLAFUSE Technology TM), the H-HA and L-HA chains, contained in PREJUVA®, interact with each other providing unique rheological characteristics and thus allowing the administration of higher concentrations of HA without increasing the viscosity.
PREJUVA® acting through a corrective/filling action of natural and induced cutaneous depressions, intervenes:
• in the physiological process of skin aging, the effects of which include reduced skin hydration, the alteration of elastic fibers and collagen of the dermis, with loss of turgor and skin tone;
• in the dermal tissue repair process, in cases of scars resulting from superficial Cutaneous trauma (e.g. acne and chicken pox scars).


Dermax Co., Ltd. is a biotechnology enterprise specialized in the research, production and sale of aesthetic products. The establishment of professional technical team, GMP production plant, complete and strict testing system has guaranteed stable and advanced manufacturing technique. The high quality, integrity and global marketing network enable our company to win high praise home and abroad including in Europe, America, Southeast Asia etc.

